Nina Sarvašová, Viola Tokárová, Ivan Saloň, František Štěpánek
The fundamental principle of targeted delivery is to secure the transport of an active substance to the desired site within the object of interest. This approach could be exploited in various types of applications ranging from crop protection to drug delivery. The adhesion of colloidal carriers to the target substrate, which can be a layer of biological cells, under the shear forces of a flowing fluid in structurally complex geometrical arrangement is therefore of interest. In our laboratory, we are focusing on the design of a combined experimental and computational method suitable for studying particle adhesion in an artificial flow system simulating the real conditions (flow rate and pore morphology). This method should serve as a platform for the in vitro evaluation of colloidal particle adhesion to biological and non-biological substrates under perfusion conditions using the combination of Magnetic Resonance Imaging (MRI) and Computational Fluid Dynamics (CFD) (Fig. 1).
Porous structures are usually fabricated by the solid template method or 3D printing. The qualitative characterization of their 3-dimensional structure and the fluid velocity profiles is performed using MRI (Fig. 2 a), and the shear rate distribution acting at the walls is evaluated from a CFD model (Fig. 2 b). During the actual measurement, a suspension of colloidal particles is introduced into the perfused 3D structures with the fraction of adhered particles evaluated as a function of the fluid flow rate (hence wall shear rate) and the porous media topology. The spatial distribution of adhered particles is simultaneously monitored by MRI. Such platform was found to be suitable for non-invasive monitoring of colloidal particle deposition in spatially complex 3D media under perfusion conditions.
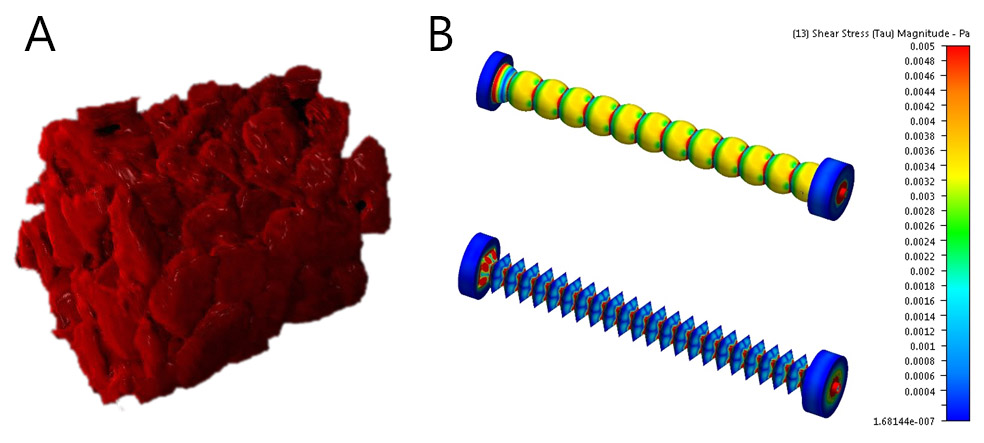
Figure 2: 3D reconstruction of perfusion layer prepared by the solid template method (a), the wall shear pressure profiles on the 2 perfusion layers with sphere (up) and octahedron (down) geometry (b).
The perfusion flow cell designed for the adhesion experiments within this laboratory allows the incorporation of substrates overgrown with tumor cell cultures expressing specific surface adhesion molecules, which can be targeted by the drug delivery system, and it can be operated with fluid velocities comparable to those encountered at both capillary level (0.1-5.0 mm/s) and in the interstitial space in tissues (10s μm/s).
Publications
Conference poster presentations:
 Sarvašová N., Majerská M., Dvořák J., Štěpánek F. The study of adhesion in 3D perfusion systems. In Sborník příspěvků z 62. konference chemického a procesního inženýrství (CHISA 2015), 2015, pp. “0109-1”-“0109-8”.
Sarvašová N., Majerská M., Dvořák J., Štěpánek F. The study of adhesion in 3D perfusion systems. In Sborník příspěvků z 62. konference chemického a procesního inženýrství (CHISA 2015), 2015, pp. “0109-1”-“0109-8”. Sarvašová N., Majerská M., Karasová A., Dvořák J., Král V., Štěpánek F. The use of MRI technology for studying the adhesion of microparticles, 29th Conference of the European Colloid and Interface Society ECIS 2015, Bordeaux, France
Sarvašová N., Majerská M., Karasová A., Dvořák J., Král V., Štěpánek F. The use of MRI technology for studying the adhesion of microparticles, 29th Conference of the European Colloid and Interface Society ECIS 2015, Bordeaux, France

