Kateřina Punčochová, Marie Prajzlerová, Nina Sarvašová, Michaela Gajdošová, František Štěpánek
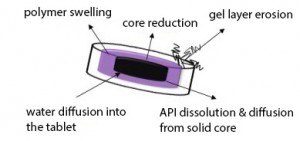
In modern medicine, sustained-release formulations offer the solution for many problems of oral drug delivery. The materials most widely used in preparing such formulations include both hydrophilic and hydrophobic polymers. The determination of the tablet structure in order to obtain the required release curve of active pharmaceutical ingredient (API) is generally time consuming process. Many tablets with different structures have to be produced and tested. However, there are only empirical approaches for simplifying the selection of matrix components known yet. Therefore, it has become necessary to fully understand and describe the processes occurring during the matrix dissolution. For this purpose, the use of imaging methods in pharmaceutical research has recently increased. A significant advantage of these methods is the possibility of real-time in situ monitoring.
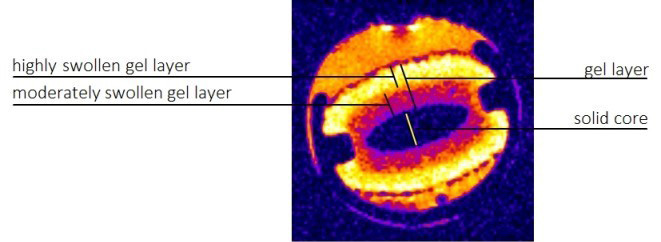
In our study, we focus on the Magnetic resonance imaging (MRI). It offers many opportunities to widen our knowledge of the drug delivery systems both in vitro (monitoring of different types of delivery systems, pharmaceutical processing) and in vivo (the studies of drug delivery systems in animals and in humans). In vitro monitoring of the dissolution of hydrophilic polymeric matrices utilizing MRI can contribute to deeper understanding of the process and lead to more proper engineering description of processes occurring during the dissolution experiments (Figure 1).
 |
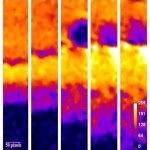 |
Figure 2: The possibilities of the evaluation of polymeric matrix swelling
The main goal of this research is to create a database of matrix components, conditions of technological processes and their effects on the dissolution profile of the API. Such database would notably simplify the formulation of dosage forms with the desired drug release profile.
Currently, our laboratory is equipped with MRI desktop system Bruker ICON™. For this scanner, flow-through cell and a plastic tablet holder have been designed and manufactured by 3D printing technology (Figure 3).
 |
 |
Figure 3: Plastic flow-through cell and tablet holder
So far, the pilot study has been carried out and MRI with the complementary methods, such as texture analysis or ATR-FTIR, proved to be the suitable means for our purposes. Future research will be focused on the evaluation of API dissolution and diffusion from the matrix, water diffusion into the tablet and the effects of matrix components on these two processes.
Publications:
- Punčochová K., Ewing, A.E., Gajdošová M., Pekárek T., Beránek J., Kazarian S.G., Štěpánek F., “The combined use of imaging approaches to assess drug release from mutlicomponent solid dispersions”, Pharm. Res. 34, 990-1001 (2017)
- Punčochová K., Prajzlerová M., Beránek J., Štěpánek F., “The impact of polymeric excipients on the particle size of poorly soluble drugs after pH-induced precipitation”, Eur. J. Pharm. Sci. 95, 138-144 (2016)
- Punčochová K., Vukosavljevic B., Hanuš J., Beránek J., Windbergs M., Štěpánek F., “Non-invasive insight into the release mechanisms of a poorly soluble drug from amorphous solid dispersions by confocal Raman microscopy”, Eur. J. Pharm. Biopharm. 101, 119-125 (2016)
- Punčochová K., Ewing A.V., Gajdošová M., Sarvašová N., Kazarian S.G., Beránek J., Štěpánek F., “Identifying the mechanisms of drug release from amorphous solid dispersions using MRI and ATR-FTIR spectroscopic imaging”, Int. J. Pharm. 483, 256-267 (2015)
- Punčochová K., Heng J.Y.Y., Beránek J., Štěpánek F., “Investigation of drug-polymer interaction in solid dispersions by vapour sorption methods”, Int. J. Pharm. 469, 159-167 (2014)
Conference poster presentations:
 Punčochová K., Vukosavljevic B., Windbergs M., Beránek J., Štěpánek F. Recrystallization of poorly soluble drug from amorphous solid dispersion recognized by confocal Raman microscopy, 6th BBBB Conference on Pharmaceutical Sciences 2015, Helsinki, Finland
Punčochová K., Vukosavljevic B., Windbergs M., Beránek J., Štěpánek F. Recrystallization of poorly soluble drug from amorphous solid dispersion recognized by confocal Raman microscopy, 6th BBBB Conference on Pharmaceutical Sciences 2015, Helsinki, Finland Punčochová K., Vukosavljevic B., Windbergs M., Hanuš J., Beránek J., Štěpánek F. Confocal Raman imaging provides evidence of a new release mechanism of poorly soluble drug from amorphous solid dispersion, 2015 AAPS Annual Meeting and Exposition, Orlando, FL, USA
Punčochová K., Vukosavljevic B., Windbergs M., Hanuš J., Beránek J., Štěpánek F. Confocal Raman imaging provides evidence of a new release mechanism of poorly soluble drug from amorphous solid dispersion, 2015 AAPS Annual Meeting and Exposition, Orlando, FL, USA Gajdošová M., Pěček D., Grof Z., Sarvašová N., Štěpánek F. Effect of Hydrophobic Inclusions on HPMC Swelling Kinetics Studied by Magnetic Resonance Imaging combined with Mathematical Modeling, 2015 AAPS Annual Meeting and Exposition, Orlando, FL, USA
Gajdošová M., Pěček D., Grof Z., Sarvašová N., Štěpánek F. Effect of Hydrophobic Inclusions on HPMC Swelling Kinetics Studied by Magnetic Resonance Imaging combined with Mathematical Modeling, 2015 AAPS Annual Meeting and Exposition, Orlando, FL, USA Punčochová K., Beranek J., Štěpánek F. Properties of the solid solutions of poorly soluble drugs prepared by hot melt extrusion and spray drying, 9th World Meeting on Pharmaceutics, Biopharmaceutics and Pharmaceutical Technology 2014, Lisbon, Portugal
Punčochová K., Beranek J., Štěpánek F. Properties of the solid solutions of poorly soluble drugs prepared by hot melt extrusion and spray drying, 9th World Meeting on Pharmaceutics, Biopharmaceutics and Pharmaceutical Technology 2014, Lisbon, Portugal Punčochová K., Beránek J., Ewing A, Kazarian S.G., Štěpánek F. Precipitation of poorly soluble drug during dissolution determined by MRI and ATR-FTIR Imaging, Poorly soluble drugs workshop 2014, Lille, France
Punčochová K., Beránek J., Ewing A, Kazarian S.G., Štěpánek F. Precipitation of poorly soluble drug during dissolution determined by MRI and ATR-FTIR Imaging, Poorly soluble drugs workshop 2014, Lille, France Punčochová K., Beránek J., Ewing A., Kazarian S.G., Štěpánek F.Investigation of dissolution mechanisms by imaging methods, AAPS Annual Meeting and Exposition 2014, San Diego, CA, USA
Punčochová K., Beránek J., Ewing A., Kazarian S.G., Štěpánek F.Investigation of dissolution mechanisms by imaging methods, AAPS Annual Meeting and Exposition 2014, San Diego, CA, USA Gajdošová M., Sarvašová N., Pěček D., Štěpánek F. Measurement of the polymer swelling kinetics: comparison of mri imaging and texture analyzer. V Proceedings of the 41st International Conference of Slovak Society of Chemical Engineering. ISBN nebo ISSN: 978-80-89475-13-1, 2014, str. 568-575.
Gajdošová M., Sarvašová N., Pěček D., Štěpánek F. Measurement of the polymer swelling kinetics: comparison of mri imaging and texture analyzer. V Proceedings of the 41st International Conference of Slovak Society of Chemical Engineering. ISBN nebo ISSN: 978-80-89475-13-1, 2014, str. 568-575.