Beyza Aysan, Jaroslav Hanuš, Marek Šoltys, Mandeep Singh, František Štěpánek
Researchers follow different synthesis routes to obtain magnetic nanoparticles (MNP’s) which can be categorised as: (i) Solid Phase Synthesis – combustion synthesis, annealing, solid state chemical reaction method; (ii) Liquid Phase Synthesis – precipitation, hydrothermal reactions, sol-gel reactions, microemulsion methods, sonolysis, polyol methods etc. (iii) Gas phase synthesis – chemical vapour deposition , arc discharge, laser pyrolysis etc. are being widely used. However, iron oxide (magnetite and maghemite) is often being utilised for biomedical applications and co-precipitation route is most commonly being followed by the researchers, as it has the advantage of being a rapid and highly efficient (in terms of yield) chemical pathway to obtain at benign conditions. To form a stable ferrofluid, the nanoparticle surface is coated either by a surfactant layer (steric stabilizers) or by inducing opposite charge (ionic stabilizers) as shown in figure.
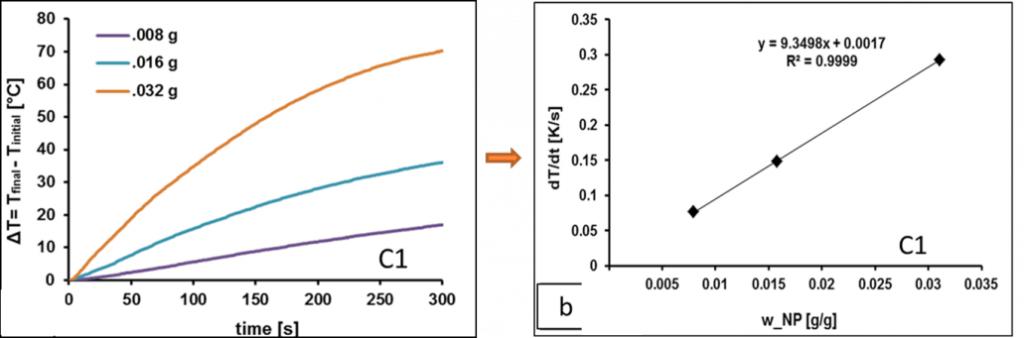
When MNP’s are exposed to an alternating magnetic field (AMF), they generate heat through oscillation of their magnetic moments, either due to Brownian relaxation (rotation of the entire magnetic particle within a surrounding medium); Neel relaxation (rotation of the magnetic moment within the magnetic core) and/or hysteresis losses. Generally, the heating power is dominated by the faster regime of relaxation. The Specific Absorption Rate (SAR) of the magnetic nanoparticles was calculated from the heating rates (ΔT/Δt) as
where mL and mNPis the mass of the dispersion medium and suspended nanoparticles, respectively, and cp,L and cp,NP are their specific heat capacities. However, our results, shows that SAR increases with increase in – NP core size; net saturation magnetization at room temperature; applied field strength and frequency and decreases with surface coating either steric or ionic. On the other hand, SAR is not much affected by viscosity, unless they are fixed within some matrix.
Publications
- Haša J., Hanuš J., Štěpánek F., “Magnetically controlled liposome aggregates for on-demand release of reactive payloads”, ACS Appl. Mater. Interfaces 10, 20306–20314 (2018)
- Aysan A.B., Knejzlík Z., Ulbrich P., Šoltys M., Zadražil A., Štěpánek F., “Effect of surface functionalisation on the interaction of iron oxide nanoparticles with polymerase chain reaction”, Coll. Surf. B 153, 69-76 (2017)
- Pittermannová A., Ruberová Z., Zadražil A., Bremond N., Bibette J., Štěpánek F., “Microfluidic fabrication of composite hydrogel microparticles in the size range of blood cells”, RSC Adv. 6, 103532-103540 (2016)
- Šoltys M., Kovačík P., Lhotka M., Ulbrich P., Zadražil A., Štěpánek F., “Radiofrequency controlled release from mesoporous silica nano-carriers”, Micropor. Mesopor. Mater. 229, 14-21 (2016)
- Zadražil A., Štěpánek F., “Remote control of reaction rate by radiofrequency heating of composite catalyst pellets”, Chem. Eng. Sci. 134, 721-726 (2015)
- Sarvašová N., Ulbrich P., Tokárová V., Zadražil A., Štěpánek F., “Artificial swarming: towards radiofrequency control of reversible micro-particle aggregation and deposition”, Powder Technol. 278, 17-25 (2015)
- Ullrich M., Hanuš J., Štěpánek F., “Remote control of enzymatic reaction in compartmentalized microparticles: a system for the delivery of unstable actives”, Chem. Eng. Sci. 125, 191-199 (2015)
- Kovačík P., Singh M., Štěpánek F., “Remote control of diffusion from magnetic hollow silica microspheres”, Chem. Eng. J. 232, 591-598 (2013)
- Zadražil A., Štěpánek F., “Remote control of desorption by radiofrequency heating: Single pellet experiments”, Chem. Eng. Sci. 101, 382-389 (2013)
- Hanuš J., Ullrich M., Dohnal J., Singh M., Štěpánek F., “Remotely controlled diffusion from magnetic liposome microgels”, Langmuir 29, 4381-4387 (2013)
- Singh M., Ulbrich P., Prokopec V., Svoboda P., Šantavá E., Štěpánek F., “Effect of hydrophobic coating on the magnetic anisotropy and radiofrequency heating of γ-Fe2O3 nanoparticles”, J. Magn. Magn. Mater. 339, 106-113 (2013)
- Singh M., Ulbrich P., Prokopec V., Svoboda P., Šantavá E., Štěpánek F., “Vapour phase approach for iron oxide nanoparticle synthesis from solid precursors”, J. Solid State Chem., accepted (2013)
- Zadražil A., Tokárová V., Štěpánek F., “Remotely triggered release from composite hydrogel sponges”, Soft Matter 8, 1811-1816 (2012)