Marek Šoltys, Sarah Akhlasová, Martin Balouch, Jakub Mužík, David Zůza, Pavel Kovačík, Josef Beránek, František Štěpánek
High number of newly discovered active pharmaceutical ingredients (APIs) are poorly soluble in water which decreases their bioavailability and makes their formulation into a functional pharmaceutical product quite a challenge. One of the possible solutions is amorphization of the API. The amorphous form is thermodynamically less stable and thus generally provides better dissolution kinetics than more stable crystalline states of the APIs. However the amorphous state tends to spontaneously crystallize over time and needs to be stabilized somehow in order to be viably formulated.
There are several methods of achieving stable amorphous formulations. One of them is formulation on porous carriers. The confined space of mesopores prevents the API from crystallizing and in combination with high surface area leads to faster release kinetics. A wide range of possible porous micro- and nano-carriers is being thouroughly studied at the moment in relation to their compatibility with specific APIs, their loading capacity and their effectivity on increasing the dissolution rate.

TEM close up picture of the porous structure of a solid silica nanoparticle with individual pores measuring 3 nm in diameter.
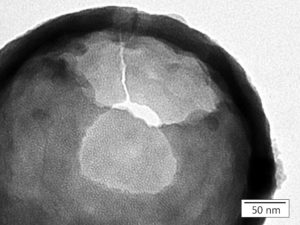
TEM close up picture of the porous structure of a hollow silica nanoparticle with individual pores measuring 3 nm in diameter.
Publications
- Šoltys M., Akhlasová S., Zadražil A., Kovačík P., Štěpánek F., “Manufacturing of multi-drug formulations with customised dose by solvent impregnation of mesoporous silica tablets”, AAPS PharmSciTech 20:25 (2019)
- Šoltys M., Kovačík P., Dammer O., Beránek J., Štěpánek F., “Effect of solvent selection on drug loading and amorphisation in mesoporous silica particles”, Int. J. Pharm. 555, 19-27 (2019)
- Zůza D., Šoltys M., Mužík J., Lizoňová D., Lhotka M., Ulbrich P., Kašpar O., Štěpánek F., “Silica particles with three levels of porosity for efficient melt amorphisation of drugs”, Micropor. Mesopor. Mater. 274, 61-69 (2019)
- Lizoňová D., Mužík J., Šoltys M., Beránek J., Kazarian S.G., Štěpánek F., “Molecular-level insight into hot-melt loading and drug release from mesoporous silica carriers”, Eur. J. Pharm. Biopharm. 130, 327-335 (2018)
- Šoltys M., Balouch M., Kašpar O., Lhotka K., Ulbrich P., Zadražil A., Kovačík P., Štěpánek F., “Evaluation of scale-up strategies for the batch synthesis of dense and hollow mesoporous silica microspheres”, Chem. Eng. J. 334, 1135-1147 (2018)
