Jan Haša, Martina Živčáková, Martin Ullrich, Jaroslav Hanuš, František Štěpánek
Liposomes are small vesicles based on phospholipids forming a bilayer. Their size usually ranges from 50 nm to several micrometers. Their hollow structure enables to encapsulate a wide spectrum of substances. Hydrophobic parts of phospholipids molecules (“tails”) make a diffusion barrier for hydrophilic substances and also can incorporate hydrophobic molecules. Liposomes are biocompatible so they have been used in medicine as carriers of drugs. Liposomes have also an important property for controlled release studies. Bilayer permeability can be modified by temperature. The bilayer melts from solid (gel) to liquid state and changes dramatically its permeability. The “melting temperature” depends on composition of the bilayer (e.g. for DPPC = dipalmitoyl(phosphatidyl)choline it is 41°C).
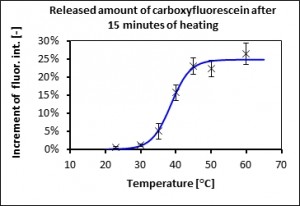
Generally we use 2 ways of determining of release kinetics. We utilize a self-quenching fluorescence dye carboxyfluorescein or reaction between enzyme laccase and ABTS = 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid). Released amount is determined fluorimetrically (resp. photometrically). Temperature change of diffusivity through the bilayer is showed in Fig.1.
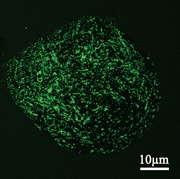
We study systems of liposomes immobilized within alginate gel particles. Sodium alginate is a substance isolated from brown algae. Calcium salt of alginate is commonly used in a food industry as a gelling agent. In our case the gel protects (chemically / physically) fragile liposomes. Confocal microscope image of the gel particle prepared by ink-jet method containing liposomes is in Fig.2. In our work we have showed how to stabilize liposomes inside the alginate gel beads and control the release of model substances by temperature. We have managed to control the release by radiofrequency heating of magnetite nanoparticles embedded within the gel..
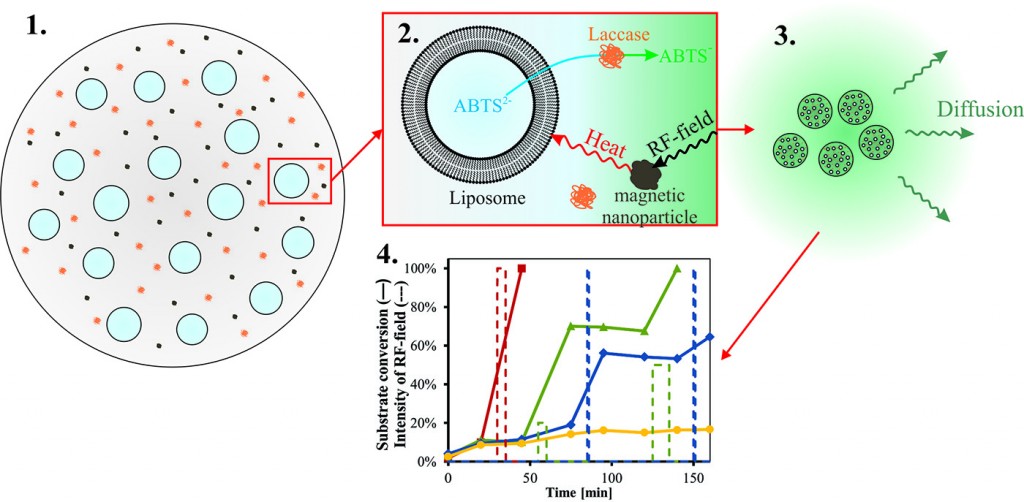
Recently we have studied such particles containing an enzyme-substrate system. Substrate ABTS was encapsulated within the liposomes and enzyme laccase was immobilized in the alginate gel. Utilizing this system we were able to remotely control the progress of the enzymatic reaction within the microparticles by radiofrequency field and thus to prepare tiny bioreactors (Fig.3).
Publications
- Haša J., Hanuš J., Štěpánek F., “Magnetically controlled liposome aggregates for on-demand release of reactive payloads”, ACS Appl. Mater. Interfaces 10, 20306–20314 (2018)
- Ullrich M., Haša J., Hanuš J., Šoóš M., Štěpánek F., “Formation of multi-compartmental particles by controlled aggregation of liposomes”, Powder Technol. 295, 115-121 (2016)
- Ullrich M., Hanuš J., Štěpánek F., “Remote control of enzymatic reaction in compartmentalized microparticles: a system for the delivery of unstable actives”, Chem. Eng. Sci. 125, 191-199 (2015)
- Ullrich M., Hanuš J., Dohnal J., Štěpánek F., “Encapsulation stability and temperature-dependent release kinetics from hydrogel-immobilised liposomes”, J. Coll. Interf. Sci. 394, 380-385 (2013)
- Hanuš J., Ullrich M., Dohnal J., Singh M., Štěpánek F., “Remotely controlled diffusion from magnetic liposome microgels”, Langmuir 29, 4381-4387 (2013)