David Smrčka, Marek Schöngut, František Štěpánek
Granulation belongs to the field of particle technology, since it deals with complex systems, where solid fraction is not homogenous but dispersed into many smaller fractions. It is a process during which are smaller particles agglomerated into larger clusters in which are original particles still recognizable. To initiate granulation is in most case needed an addition of a binder, which is a substance with high adhesive properties allowing particles to stick together.
A common method for characterization of granulated materials is size distribution, i.e. relative amount of different size fractions in given material. Particle size distribution (PSD) can be obtained various ways, from the simplest describing just the size (sieving analysis), to more complex capable of description of size, shape etc. (image analysis or laser diffraction).
Yet despite all the progress that has been made in the particle technology field since the 50s, granulation still remains more of an art based on empirical knowledge than a science field with robust engineering background. A slight change in formulation or process parameters (change of primary particle size or in ambient temperature respectively) frequently leads to a product with different properties, often undesired.
In our research group based at the Institute of Chemical Technology in Prague high-shear wet granulation process is intensively investigated. In cooperation with Zentiva k.s. complete pharmaceutical formulation with a high load of API is being subjected to a comprehensive study of the process scale-up. That includes parametric study on a laboratory scale to obtain an impression about parametric sensitivity of produced granules. Since the dissolution curves are considered as a criterion describing product quality, similarity (based on calculation of similarity factor for two curves) of dissolution profiles is being preferably used for assessment of successful scale-up. Based on literature and proved by experiments, Froude number is established as a reliable scaling parameter for maintaining similar dissolution profiles during scale-up from laboratory (0.5 and 4L granulator) onto semi-manufacturing scale (25L granulator) and manufacturing scale (400L granulator).
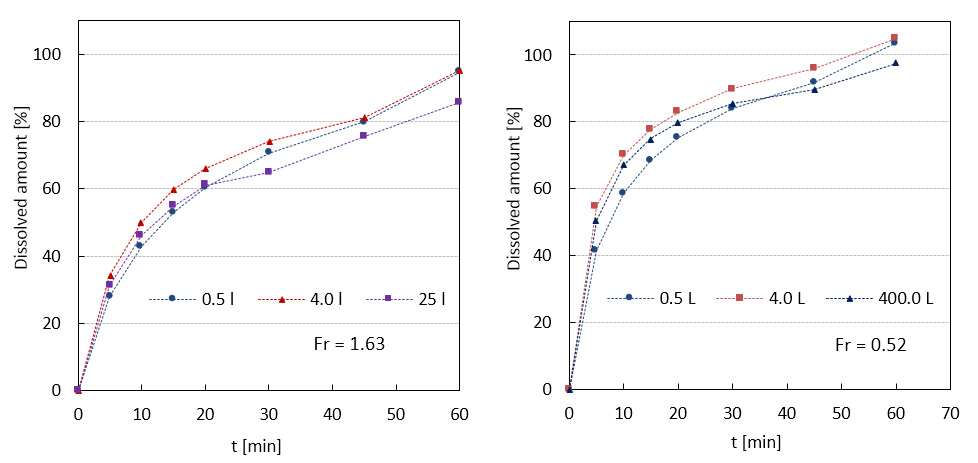
Comparison of dissolution profiles from two scale-up studies (0.5 to 4 to 25 L) and (0.5 to 4 to 400 L).
For deeper insight into dissolution of granules, methodology facilitating simultaneous measurement of granule disintegration and dissolution is being developed by combination of online measurement of PSD on a laser size analyzer using a static light scattering and offline measurement an amount of dissolved API on UV-vis spectrophotometer.
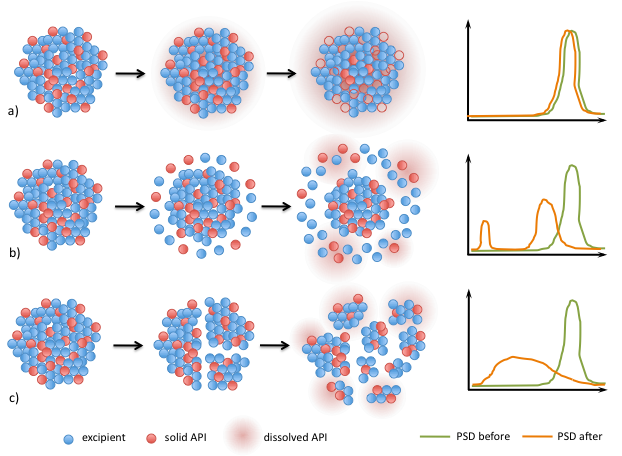
Different release mechanisms (a) leaching, b) surface erosion and c) breakage) of API from granules are proposed and are being established by experiments.
Granule inner structure is being investigated by micro-CT (computational tomography) analysis usinga SkyScan micro-CT attachment for scanning electron microscope with a CCD detector.
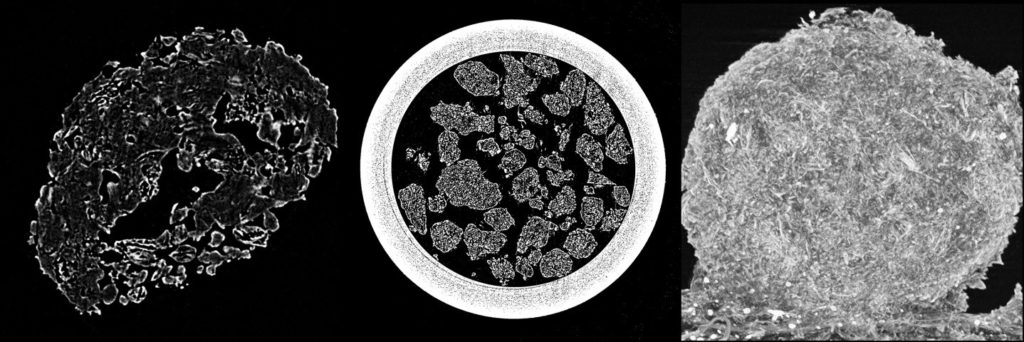
CT scans of granules showing their inner structure
Publications
- Kataria A., Oka S., Smrčka D., Grof Z., Štěpánek F., Ramachandran R., “A quantitative analysis of drug migration during granule drying”, Chem. Eng. Res. Des. in press (2018)
- Smrčka D., Lesáková V., Dohnal J., Grof. Z., Štěpánek F., “Experimental analysis of inter- and intra-batch variation of granule porosity, stiffness and dissolution rate”, Chem. Eng. Res. Des. 132, 1131-1142 (2018)
- Oka S., Smrčka D., Kataria A., Emady H., Muzzio F.J., Štěpánek F., Ramachandran R., “Analysis of the origins of content non-uniformity in high-shear wet granulation”, Int. J. Pharm. 528, 578-585 (2017)
- Smrčka D., Dohnal J., Štěpánek F., “Dissolution and disintegration kinetics of high-active pharmaceutical granules produced at laboratory and manufacturing scale”, Eur. J. Pharm. Biopharm., in press (2016)
- Adepu M., Hate S., Bétard A., Oka S., Schöngut M., Sen M., Sood Y., Wolf D., Wieland S., Stepanek F., Muzzio F., Glasser B., Ramachandran R., “Quantitative validation and analysis of the regime map approach for the wet granulation of industrially relevant zirconium hydroxide powders”, Powder Technol. 294, 177-184 (2016)
- Smrčka D., Dohnal J., Štěpánek F., “Effect of process scale-up on the dissolution of granules with a high content of active pharmaceutical ingredient”, Powder Technol. 285, 88-95 (2015)
- Chaudhury A., Tamrakar A., Schöngut M., Smrčka D., Štěpánek F., Ramachandran R., “Multi-dimensional population balance model development and validation of a reactive detergent granulation process”, Ind. Eng. Chem. Res. 54, 842–857 (2015)
- Schöngut M., Smrčka D., Gregor T., Štěpánek F., “Investigation of rate-limiting steps during granulation with a chemically reactive binder”, Powder Technol. 270, 510-519 (2015)
- Smrčka D., Schöngut M., Gregor T., Štěpánek F., “Composition limits in granulation with active component in the binder“, AIChE J. 61, 395-406 (2015)
- Schöngut M., Smrčka D., Štěpánek F., “Experimental and theoretical investigation of the reactive granulation of sodium carbonate with dodecyl-benzenesulfonic acid”, Chem. Eng. Sci. 86, 2-8 (2013)
- Kašpar O., Tokárová V., Oka S., Sowrirajan K., Ramachandran R., Štěpánek F., “Combined UV/Vis and micro-tomography investigation of acetaminophen dissolution from granules”, Int. J. Pharm. 458, 272-281 (2013)
- Schöngut M., Grof Z., Štěpánek F., “Kinetics of dry neutralization of dodecyl-benzenesulfonic acid with respect to detergent granulation”, Ind. Eng. Chem. Res. 50, 11576–11584 (2011)
