Chobotix project proposals
Whether you choose one of the topics, have a project idea of your own, or are simply curious to see how our lab works, you are always welcome here! Reach out to us at Petr.Fatka@vscht.cz to arrange a guided tour of the entire laboratory.
Click here to download pdf with all topics.
If the project you were interested in has already been CLAIMED, don’t worry! You’re welcome to reach out to the project leader. Together, you can explore creating something similar or even develop a completely new idea tailored just for you!
Vanluchene Lab
Laboratory of Chemical Engineering for Green Chemistry
This research is focusing on sustainable advancements in fine chemical synthesis by integrating chemical engineering with heterogeneous photocatalysis and photochemistry. We prioritize using earth-abundant, metal-free catalysts, such as graphitic carbon nitride g-C3N4), and visible light as an energy source to drive eco-friendly, cost-effective chemical processes. Due to limited light penetration, this approach cannot be used in traditional batch setups, which is why we are focusing on developing novel photo microreactors.
By overcoming challenges like light penetration and solid handling in microflow reactors, we aim to revolutionise the production of active pharmaceutical ingredients and fine chemicals, achieving better sustainability, competitiveness, and reduced foreign dependency in these productions. We focus from synthesis of various graphitic carbon nitrides, development of different microreactors (microfluidic chips, flow cells) to study mass transport phenomena in these type of reactors.
If you have an interest in any of my research topics or would like to learn more about the laboratory, please feel free to contact Anna Vanluchene at Anna.Vanluchene@vscht.cz
Nanoparticle production and drug delivery systems
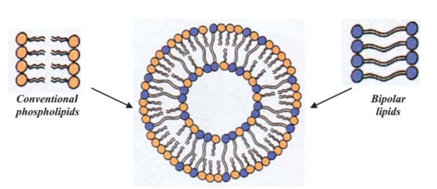
Structure of a liposome composed of conventional and bipolar lipids
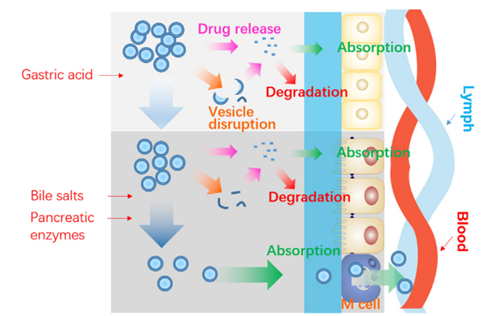
Schematic presentation of the fate of liposomes following oral administration
He, Haisheng et al. “Adapting liposomes for oral drug delivery. Acta pharmaceutica Sinica. B vol. 9,1 (2019): 36-48. doi:10.1016/j.apsb.2018.06.005
This project will investigate the effect of the tetraether lipid Glycerol dialkyl glycerol tetraether (GDGT) on the stability of liposomes. In contrast to conventional phospholipids, GDGT is composed of two polar heads linked by 2 long carbonyl chains (Fig. 2) and is thus a potential ideal candidate for increasing the stability of liposomes in harsh environments. This work will aim to prepare liposomes containing GDGT and compare their properties with liposomes containing other stability enhancing elements (cholesterol, etc.). Subsequently, 5(6)-carboxyfluorescein will be encapsulated in the liposomes, the encapsulated amount will be determined and the leakage of the substance from the liposomes will be investigated.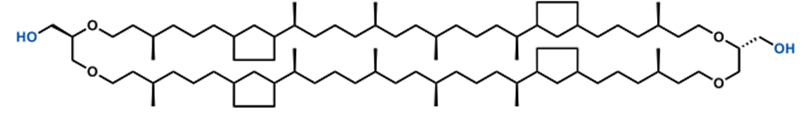
Molecular structure of GDGT
| Project Type: | Bc, Ing |
| Project Leader: | Ing. Martin Roudný |
| Contact: | Martin.Roudny@vscht.cz |
This project focuses on developing stable pharmaceutical dosage forms using wet and dry granulation techniques, with the goal of stabilising the active ingredient and preventing impurity formation. The work involves preparing granules of the active ingredient with protective coatings, followed by detailed analysis using methods like Scanning Electron Microscopy (SEM) and X-Ray Powder Diffraction (XRPD). These granules will then be compressed into tablets, which will undergo stress and stability studies to evaluate their performance under various conditions.
The stability and purity of the active ingredient will be assessed using High-Performance Liquid Chromatography (HPLC), which will help to identify any impurities or degradation products formed over time. This project offers students practical experience in pharmaceutical formulation and stability analysis, ideal for those interested in applied pharmaceutical science.
| Project Type: | Bc, Ing |
| Project Leader: | Ing. Zuzana Hlavačková |
| Contact: | Zuzana.Hlavackova@vscht.cz |
| Project Type: | Bc, Ing |
| Project Leader: | Ing. Stanislav |
| Contact: | Stanislav.Chvila@vscht.cz |
In this project, we aim to connect fundamental biophysical science with real-world pharmacology: The objective is to understand the effects of different pharmaceutical molecules on the properties of phospholipid membranes, as knowledge of these effects could explain some drug interactions in patients.
You will prepare liposomes (nanometer-sized bilayer structures composed of polar lipids) loaded with selected pharmaceuticals and measure the rate at which fluorescent dyes can cross such membranes.
What’s in there for you: an opportunity to learn the techniques for preparation and characterization of liposomes, together with advanced analytical instrumentation. We can provide a fully equipped nano-engineering lab, and a great deal of supportive mentoring.What you should bring: a willingness to learn and to try new techniques hands-on, and an open mind for curious questions and new hypotheses.
| Project Type: | Bc, Ing |
| Project Leader: | Bc. Adam Tywoniak, M.Sc. |
| Contact: | Adam.Tywoniak@vscht.cz |
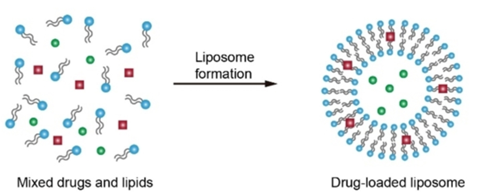
Illustration of passive loading of liposomes
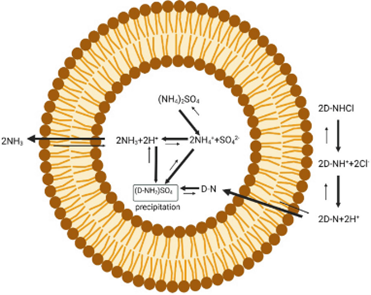
Active loading of doxorubicin into liposomes
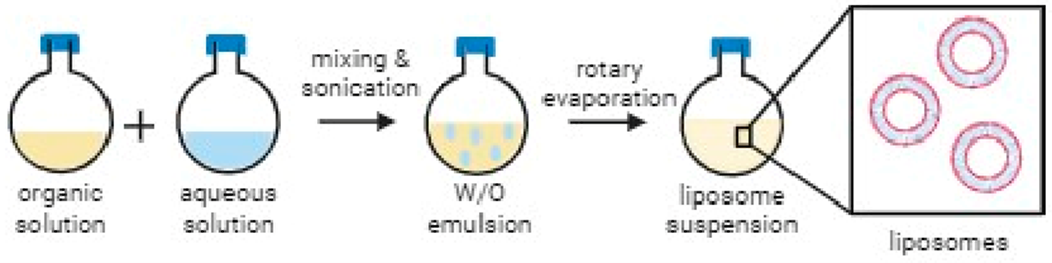
Liposome preparation via reverse phase evaporation
| Project Type: | Bc, Ing |
| Project Leader: | Ing. Martin Roudný |
| Contact: | Martin.Roudny@vscht.cz |
The field of pharmaceutical research has undergone significant progress in recent years. These advances, however, come at the cost of substantial losses due to failed drug products. This often occurs even after candidate drugs prove their efficacy in the initial stages of cell tests with solubilising agents, such as cyclodextrins. It would therefore be beneficial to establish a reliable platform for efficacy tests of novel drugs that utilises a consistent formulation approach across all stages of drug development and clinical trials.
As proof of concept, the Chobotix lab has focused on the production of phospholipid-stabilised drug nanocrystals. These have so far been prepared by wet-stirred milling with zirconium oxide beads at scales of dozens of milligrams per batch. Despite the advances made, the capabilities of small-scale milling are still limited due to loss of drug during milling and relatively long milling times in comparison to commercial apparatuses. This issue becomes even more glaring if there is only a small amount of drug available, which is common during mass synthesis screenings. Therefore, great progress can still be made in terms of improving the milling procedure. The work package can be adapted to a bachelor or to a master thesis. No previous knowledge of lab practices is necessary (while still an advantage). Some manual dexterity will be considered beneficial, as well as knowledge and application of basic principles of physics and chemical engineering.
| Project Type: | Bc, Ing |
| Project Leader: | Ing. Stanislav Chvíla |
| Contact: | Stanislav.Chvila@vscht.cz |
- Study of Glucan particles macrophage uptake, its extent, and kinetics
- Study of effect of surface modifications of GPs on their macrophage uptake
- Investigation of encapsulated APIs effect on the uptake
| Project Type: | Bc, Ing |
| Project Leader: | Ing. Petr Fatka |
| Contact: | Petr.Fatka@vscht.cz |
Computational and AI techniques in drug analysis
| Project Type: | Bc, Ing |
| Project Leader: | Ing. Terezie Císařová |
| Contact: | Terezie.Cisarova@vscht.cz |
One of the approaches to work around low solubility of active pharmaceutical compounds are self-emulsifying drug delivery systems comprising a lipid carrier and surfactants. Development of such systems is largely empirical, and therefore, automation of the process is currently being explored with a robotic pipetting system. Moreover, AI assistance could be beneficial to reduce the amount of required experimental work.
- One Bc. or Ing. student project would be focused on developing a neural network aimed at predicting the optimal composition of a self-emulsifying drug delivery system for a given active compound, machine learning on a dataset created by the robotic pipetting system. CLAIMED
- Another Bc. or Ing. project would be aimed on optimising the robotic pipetting system, automating the existing screening process further with the use of a robotic arm, and exploring the influence of the active pharmaceutical ingredient on the emulsification properties.
- A third potential project for a Bc. student would explore the use of particle-stabilised (Pickering) emulsions in pharmaceutical drug development. The main envisioned steps are identification of suitable materials, preparing emulsions, drying the emulsions and assessing their redispersibility, and exploring mass transfer between the encapsulated liquid and the surrounding continuous phase.
| Project Type: | Bc, Ing |
| Project Leader: | Ing. Martin Krov |
| Contact: | Martin.Krov@vscht.cz |
Crystallisation is the most significant separation and purification process in the production of solid substances, widely used in fields such as pharmaceuticals, food production, and agriculture. In addition to classic crystallisation techniques, such as evaporative and cooling crystallisation, crystals can also be generated by methods that involve mixing fluids of differing compositions. These methods include continuous and semi-batch processes, where supersaturation is induced either by chemical reaction or by altering solubility through the addition of an antisolvent. This project focuses on investigating these specific processes.
The main issue is that the modelling of such systems generally assumes ideal mixing, which can often lead to significant inaccuracies. Literature indicates that, as mixing intensity increases, crystal size may increase, decrease, or reach a maximum or minimum, yet the underlying reasons for these phenomena remain unclear. This project aims to address these gaps. We will work with an existing crystallisation model that accounts for non-ideal mixing. The objective is to conduct experimental parametric studies on various crystalline substances, both inorganic and organic, across different types of crystallisers. Based on these data, we will validate, and if necessary, extend the crystallisation model to explore the interaction between mixing and crystallisation through simulations.
| Project Type: | Bc, Ing |
| Project Leader: | Ing. Jan Trnka |
| Contact: | Jan.Trnka@vscht.cz |
Pharmaceutical production methods
| Project Type: | Bc, Ing |
| Project Leader: | Ing. Stanislav Chvíla |
| Contact: | Stanislav.Chvila@vscht.cz |
This project aims to advance methodologies for permeability measurement in pharmaceutical applications using an in-house, custom-developed device capable of simultaneous dissolution and permeation assessment. There are two main areas of focus:
-
Engineering and Application Development
The first aspect concentrates on engineering the device and exploring its applications, specifically investigating how permeability is influenced by factors such as pH, hydrodynamic conditions, and molecular size. The goal is to gain insight into how these variables affect permeability measurements and improve the reliability of in vitro-in vivo correlation (IVIVC) predictions. -
Bio-membrane Development and Alternative Permeability Measurement Approaches
The second part of the project focuses on bio-membrane innovation, exploring advanced methodologies such as measuring permeability using living cell cultures. This research aims to better replicate biological conditions, improving the relevance and accuracy of permeability measurements in a pharmaceutical context.
Both parts of this project demand interdisciplinary collaboration and innovative, out-of-the-box thinking. The outcome is expected to significantly impact the understanding and prediction of permeability behaviours, contributing to enhanced drug development processes.
| Project Type: | Bc, Ing |
| Project Leader: | Ing. David Zůza |
| Contact: | David.Zuza@vscht.cz |
Glucan particles (GPs) derived from baker’s yeast (Saccharomyces cerevisiae) have shown promise as vaccine adjuvants and bioactive carriers for targeted drug delivery. However, the current preparation method is restricted to laboratory-scale production, involving batch processing; a high volume of organic solvents relative to the GPs produced, and limited output. These constraints pose challenges to the industrial scalability of GPs.
In this project, we seek to examine the current GP preparation method and develop a more efficient and scalable process that supports continuous production. For that, we aim to develop an innovative process based on centrifugation with filtration technology. We will be testing the new equipment and developing the necessary modifications with the help of 3D printing.
| Project Type: | Bc, Ing |
| Project Leader: | Gabriela Ruphuy Chan, M.Sc., Ph.D. |
| Contact: | ruphuycg@vscht.cz |
| Project Type: | Bc, Ing |
| Project Leader: | Ing. Filip Zavřel |
| Contact: | Filip.Zavrel@vscht.cz |
| Project Type: | Bc, Ing |
| Project Leader: | Ing. Stanislav Chvíla |
| Contact: | Stanislav.Chvila@vscht.cz |
Vanluchene lab projects
Direct oxygenation of C-H bonds is atom-economical but limited by oxygen’s low reactivity under mild conditions. Singlet oxygen, being more reactive, is valuable for pharmaceutical synthesis, yet batch processes suffer from poor irradiation and short singlet oxygen lifespans. Flow reactors address this limitation, and using metal-free g-C3N4 photocatalysts adds a sustainable dimension. This project will focus on:
- Immobilisation of photocatalyst g-C3N4 on glass.
- Construction of a microflow cell for enhanced photochemical processes.
- Evaluation of singlet oxygen generation efficiency using UV/VIS.
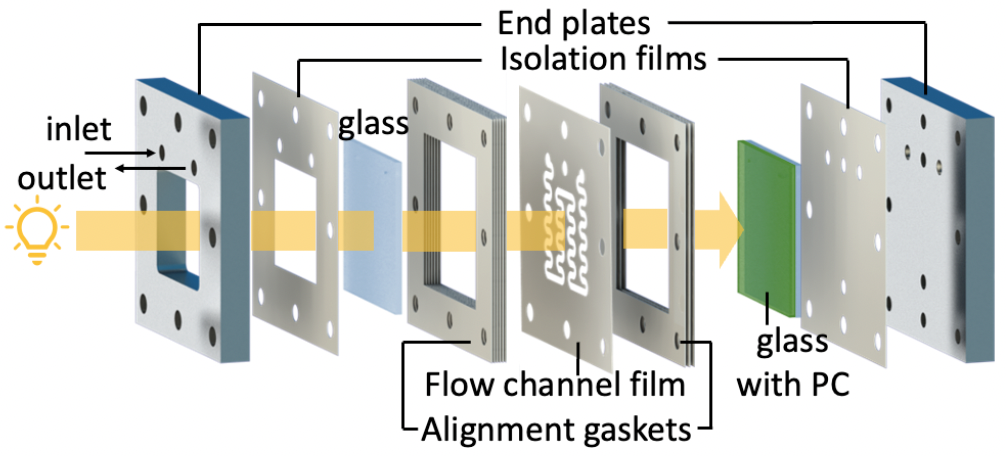
Graphitic carbon nitride (g-C3N4) is an inexpensive, metal-free photocatalyst with activity in the visible light spectrum. This project aims to synthesize and thoroughly characterize different types of carbon nitride and investigate the effects of doping with nanoparticles, such as Fe2O3. The synthesized materials will be fully characterized, and their photocatalytic activity will be tested. Thin layers of the doped carbon nitride will be prepared and evaluated for their photocatalytic performance. Additionally, the impact of plasma treatment on the surface of these thin layers and its influence on photocatalytic efficiency and stability will be explored.
- Synthesis and characterization of various photocatalysts.
- Preparation of thin layers of photocatalyst and evaluation of their photocatalytic activity.
- Exploration of plasma treatment effects on surface properties, photocatalytic efficiency, and stability of thin layers.
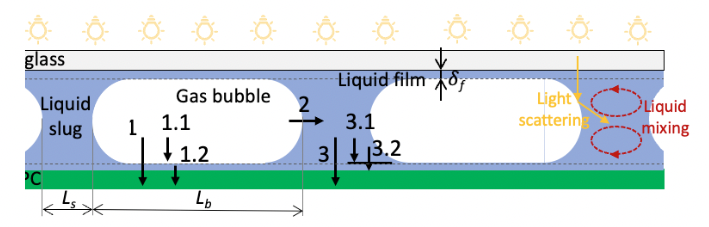
- Immobilisation of photocatalyst g-C3N4 in microfluidic chips.
- Fabrication of microfluidic chips using soft lithography as a 4-phase reactor (gas/liquid/solid/photons).
- Optimization of mass transfer and photon utilization in the microfluidic chip.
